Which of the Following Would Lead You to Suspect the Patient s Stroke is Continuing to Get Worse
Continuing Education Activity
Cerebrovascular accidents, commonly known as strokes, are prevalent across patient populations and can be a significant cause of morbidity and mortality. Strokes can be categorized as ischemic, hemorrhagic, or subarachnoid. This activity reviews the evaluation and management of ischemic cerebrovascular accidents and highlights the role of the interprofessional team in the recognition and management of this condition.
Objectives:
-
Review the pathophysiology of ischemic cerebrovascular accidents.
-
Describe the typical presentation of a patient with an ischemic cerebrovascular accident.
-
Describe the recommended management of ischemic cerebrovascular accidents, including immediate interventions, mitigation of sequelae, and subacute management.
-
Explain interprofessional team strategies for improving care coordination and communication regarding the management of patients affected by acute and subacute cerebrovascular accidents.
Access free multiple choice questions on this topic.
Introduction
Stroke, a cerebrovascular accident, is prevalent across patient populations and can be a significant cause of morbidity and mortality. Strokes can be categorized as ischemic, hemorrhagic, or subarachnoid. Among ischemic strokes, the Trial Org 10172 in Acute Stroke Treatment (TOAST) classification is used to subdivide the categories that include cardioembolism, small-vessel occlusion, large-artery atherosclerosis, and stroke of undetermined etiology.[1]
Etiology
The etiology of ischemic stroke is due to either a thrombotic or embolic event that causes a decrease in blood flow to the brain. In a thrombotic event, the blood flow to the brain is obstructed within the blood vessel due to dysfunction within the vessel itself, usually secondary to atherosclerotic disease, arterial dissection, fibromuscular dysplasia, or inflammatory condition. In an embolic event, debris from elsewhere in the body blocks blood flow through the affected vessel. The etiology of stroke affects both prognosis and outcomes.[2][3]
Epidemiology
Stroke is the fifth most commonest cause of death if considered separately from other cardiovascular diseases. In the United States, an estimated 795,000 patients suffer from stroke annually, and the prevalence of stroke escalates with age. The lifetime risk of all types of stroke is higher in women; however, this is attributed to longer life expectancy.
According to the Framingham Heart Study, stroke incidence is declining over time. However, the cohort was predominantly a white population [4][5][6].
Pathophysiology
In thrombosis, there is an obstructive process that prevents blood flow to some regions of the brain. Risk factors include atherosclerotic disease, vasculitis, or arterial dissection.
Embolic events occur when there is a clot that originated from another location in the body. Most commonly, the source of the clot is the valve or chambers of the heart, for example, when a clot forms within the atria in atrial fibrillation and dislodges into the arterial vascular supply.
Other less frequent causes include venous, septic, air, or fat emboli. Lacunar infarcts are usually seen in the subcortical areas of the brain and occur due to small vessel disease. The proposed mechanism is a perforating artery in the subcortical region that causes the blood vessel occlusion.
Ischemic Stroke Syndromes
Ischemic strokes can present in pre-determined syndromes due to the effect of decreased blood flow to particular areas of the brain that correlate to exam findings. This allows clinicians to be able to predict the area of the brain vasculature that can be affected.
Middle Cerebral Artery (MCA) Infarction
The middle cerebral artery (MCA) is the most common artery involved in stroke. It supplies a large area of the lateral surface of the brain and part of the basal ganglia and the internal capsule via four segments (M1, M2, M3, and M4). The M1 (horizontal) segment supplies the basal ganglia, which is involved in motor control, motor learning, executive function, and emotions. The M2 (Sylvian) segment supplies the insula, superior temporal lobe, parietal lobe, and inferolateral frontal lobe.
The MCA distribution involves the lateral cerebral cortex. MCA syndrome is best explained by the understanding of the somatosensory cortex, in which the lateral portion contains motor and sensory functions that involve the face and upper extremity. This correlates to the classical presentation of contralateral hemiparesis, facial paralysis, and sensory loss in the face and upper extremity. The lower extremity may be involved, but upper extremity symptoms usually predominate. Gaze preferences towards the side of the lesion may be seen. Additional symptoms include:
-
Dysarthria is characterized by difficulty phonating words due to the physical weakness of the muscles of the face used for phonation.
-
Neglect where the patient seems to "ignore" a hemisphere of their world due to an inability to see that area.
-
Aphasia or the inability to produce or remember words due to injury to the verbal centers of the brain.
Anterior Cerebral Artery (ACA) Infarction
The anterior cerebral artery (ACA) provides blood supply to the frontal, prefrontal, primary motor, primary sensory, and supplemental motor cortices. Pure ACA infarcts are uncommon because of the significant collateral blood supply provided by the anterior circulating artery. The sensory and motor cortices receive sensory information and control movement of the contralateral lower extremity. The supplemental motor area contains the Broca area, which is involved in the initiation of speech. The prefrontal cortex is used to organize and plan complex behavior and is thought to influence the personality.
The ACA distribution involves the medial cerebral cortex. The somatosensory cortex in that area comprises motor and sensory functions of the leg and foot. The clinical presentation of an ACA infarction includes contralateral sensory and motor deficits in the lower extremity. The upper extremity and face are spared. Kumral et al. examined clinical spectrums of ACA with correlation to MRI/MRA and demonstrated that left-sided lesions presented with more transcortical motor aphasia, in which patients have difficulty responding spontaneously with speech, but repetition is preserved. Right-sided lesions presented with a more acute confusional state and motor hemineglect (unilateral motor function is lost) [7].
Posterior Cerebral Artery (PCA) Infarction
The superficial posterior cerebral artery (PCA) supplies the occipital lobe and the inferior portion of the temporal lobe, while the deep PCA supplies the thalamus and the posterior limb of the internal capsule, as well as other deep structures of the brain. The occipital lobe is the location of the primary and secondary visual areas, where sensory input from the eyes is interpreted. The thalamus relays information between the ascending and descending neurons, while the internal capsule contains the descending fibers of the lateral and ventral corticospinal tracts.
PCA infarctions can be divided into deep and superficial categories, based on the PCA supply. If the deep segments of the PCA are involved, symptoms may include hypersomnolence, cognitive deficits, ocular findings, hypoesthesia, and ataxia. Ocular findings may include homonymous hemianopsia, in which patients experience visual field deficits in one-half of their visual field. Larger infarcts that involve the deep structures can lead to hemisensory loss and hemiparesis due to the involvement of the thalamus and the internal capsule. Superficial infarcts present with visual and somatosensory deficits, which can include impairment of stereognosis, tactile sensation, and proprioception. Rarely, bilateral PCA infarcts present with amnesia and cortical blindness. Cortical blindness is due to lesions in the optic radiation that causes vision loss. A unilateral headache is a common finding, which can be confused with a complicated migraine. [8][9]
Vertebrobasilar Infarction
The vertebrobasilar region of the brain is supplied by the vertebral arteries and the basilar arteries that originate within the spinal column and terminate at the Circle of Willis. These areas supply the cerebellum and brainstem.
The clinical presentation includes ataxia, vertigo, headache, vomiting, oropharyngeal dysfunction, visual-field deficits, and abnormal oculomotor findings. Patterns of clinical presentation vary depending on the location and the infarction pattern of embolism or atherosclerosis. [10][11]
Cerebellar Infarction
Patients may present with ataxia, nausea, vomiting, headache, dysarthria, and vertigo symptoms. Edema and rapid clinical deterioration can complicate cerebellar infarction.
Lacunar Infarction
Lacunar infarcts result from the occlusion of a small perforating artery. The exact mechanism is under debate, as the nature of the infarct can result from intrinsic vessel occlusion or an embolism. Infarction in this territory can present with pure motor or sensory loss, sensorimotor deficit, or ataxia with hemiparesis. [12][13]
History and Physical
Ischemic strokes present acutely, and establishing the time of symptom onset is critical. If the time of symptom onset is unknown, the time the patient was last known to be normal without new neurological symptoms is used. The time that is established is then utilized to decide whether giving intravenous thrombolytics is indicated or not.
A neurological exam should be performed for all patients suspected of stroke. The National Institutes of Health Stroke Scale (NIHSS) is most commonly used to measure the severity of the stroke and has 11 categories and a score that ranges from 0 to 42. The 11 categories include the level of consciousness (LOC), which incorporates LOC questions evaluating best gaze, visual, facial palsy, motor arm, motor leg, limb ataxia, sensory, best language, dysarthria, and extinction and inattention. The stroke scale should be performed in the order listed. Each score is based on the patient's action on the exam, and it is not a prediction of what the patient can do.
The vertebrobasilar arterial (VBA) system supplies blood to the brainstem, cerebellum, and peripheral labyrinths. Occlusion of the system, therefore, can result in either central or peripheral vertigo, depending on the specific artery affected. Occlusion can occur as a result of an embolism (e.g., cardioembolism or plaque from the vertebral arteries) and may result in an ischemic infarct. Central vertigo is more commonly associated with vertical nystagmus (rather than rotational) and is typically worse with attempted gaze fixation. Peripheral vertigo often improves with gaze fixation. Additionally, dizziness associated with central vertigo is multidirectional and may change with altered gaze direction, while peripheral vertigo-associated nystagmus is unidirectional. [14]
Evaluation
An organized stroke protocol is highly recommended to expedite evaluation. The door-to-needle time of 60 minutes is recommended for acute ischemic strokes for patients who qualify for thrombolytics.
The initial evaluation of any patient is airway, breathing, circulation, and vital signs. Patients may present with respiratory abnormalities from elevated intracranial pressure and are at risk of aspiration and asphyxiation. Endotracheal intubation may be necessary to ensure adequate oxygenation and ventilation.
A fingerstick glucose check should be performed, as it is an easy way of ruling out hypoglycemia as a cause of neurological abnormalities.
A plain CT head or brain MRI is recommended for patients within 20 minutes of presentation to rule out hemorrhage. In hospitals that are stroke centers or can provide emergency care, vascular imaging should be considered for possible endovascular intervention; however, this should not delay the administration of thrombolytics.
Acute ischemic stroke can be seen on diffusion-weighted MR sequence. The FLAIR sequence helps in predicting the time since the onset of the stroke which is of paramount importance in planning thrombolytic therapy in the patient. The DWI features with no changes in the FLAIR sequence suggest the ischemic stroke is less than 6 hours old and therefore a candidate for early intravenous thrombolysis to revert the neurological deficit.
Other diagnostic tests include an electrocardiogram (ECG), troponin, complete blood count, electrolytes, blood urea nitrogen (BUN), creatinine (Cr), and coagulation factors. An ECG and troponin are suggested because stroke is often associated with coronary artery disease. A complete blood count can look for anemia or suggest infection. Electrolyte abnormalities should be corrected. BUN and Cr should be monitored as contrast studies may worsen kidney function. Coagulation factors, including PTT, PT, and INR, should also be done as the elevated levels can suggest a cause of hemorrhagic stroke.
For institutions without expert imaging interpretation, the US Food and Drug Administration highly recommends the teleradiology system for image interpretation for suspected stroke patients. This helps with the decision to administer IV alteplase. A discussion and agreement between telestroke neurologists and radiologists are highly recommended.
In areas that do not have an in-house stroke team or telestroke protocol, a telephone consultation may be considered for the administration of thrombolytics. The level of evidence is limited for this recommendation. [15][16][17]
Treatment / Management
The goal of therapy in acute ischemic stroke is to preserve tissue in areas where perfusion is decreased but sufficient to avoid infarction. Tissue in this area of oligemia is preserved by restoring blood flow to the compromised regions and improving collateral flow. Recanalization strategies include recombinant tissue-type plasminogen activator. Restoring blood flow can minimize the effects of ischemia only if performed quickly.
Endovascular techniques have been used in the treatment of acute ischemic stroke. Carotid endarterectomy has been but there is no evidence that supports its use in acute ischemic stroke. Another consideration is neuroprotective agents but none so far have been shown to improve clinical outcomes.
The following may be considered:
Alteplase
The AHA/ASA recommends intravenous (IV) alteplase for patients who satisfy inclusion criteria and have symptom onset or last known baseline within 3 hours. IV alteplase is 0.9 mg/kg, with a maximum dose of 90 mg. The first 10% of the dose is given over the first minute as a bolus, and the remainder of the dose is given over the next 60 minutes. The time has been extended to 4.5 hours for selected candidates.
Inclusion criteria include diagnosis of ischemic stroke with "measurable neurological deficit," symptom onset within 3 hours before treatment, and age 18 years or older.
A review of the exclusion criteria for thrombolytics should be performed before administering alteplase. According to the Food and Drug Administration, the contraindications to intravenous thrombolysis include active internal bleeding, recent intracranial surgery or serious head trauma, intracranial conditions that may increase the risk of bleeding, bleeding diathesis, severe uncontrolled hypertension, current intracranial hemorrhage, subarachnoid hemorrhage, and a history of a recent stroke.
For patients that present between 3 and 4.5 hours from symptoms onset, the treatment benefits and risks must be considered. Additional relative exclusion criteria for this patient category include age more than 80 years, NIHSS greater than 25, oral anticoagulant use, and a history of both diabetes and prior ischemic stroke.
Orolingual angioedema is a potential side effect of IV alteplase. If angioedema should occur, the management of the airway is a priority. Endotracheal intubation or awake fiberoptic intubation may be necessary to secure the airway. If there is suspected angioedema, hold IV alteplase and ACE inhibitors. Administer methylprednisolone, diphenhydramine, and ranitidine or famotidine. Epinephrine may be considered if the previous therapies do not alleviate signs and symptoms. Icatibant or C1 esterase inhibitor may be considered for the treatment of hereditary angioedema and ACE inhibitor angioedema.
Other fibrinolytic agents, such as tenecteplase, may be considered as an alternative to alteplase. In one study, tenecteplase appeared to have similar efficacy and safety profiles in a mild stroke but did not demonstrate superiority when compared to alteplase [18][19].
Mechanical Thrombectomy
The use of mechanical thrombectomy should be considered in all patients, even in those who received fibrinolytic therapy. The AHA/ASA guidelines do not recommend observation for a response after IV alteplase in patients who are being considered for mechanical thrombectomy.
In recent years there are significant advancements in acute stroke care. Multiple stroke trials in 2015 showed that endovascular thrombectomy in the first six hours is much better than standard medical care in patients with large vessel occlusion in the arteries of the proximal anterior circulation. These benefits are sustained irrespective of geographical location and patient characteristics.[20]
Again in 2018, a significant paradigm shift happened in stroke care. DAWN trial showed significant benefits of endovascular thrombectomy in patients with large vessel occlusion in the arteries of the proximal anterior circulation. This trial extended the stroke window up to 24 hours in selected patients using perfusion imaging. Subsequently, now more patients can be treated, even up to 24 hours.[21]
The current recommendation in selected patients with large vessel occlusion with acute ischemic stroke in the anterior circulation and who also meet other DAWN and DEFUSE 3 criteria, mechanical thrombectomy is recommended within the time frame of 6 to 16 hours of last known normal. In selected patients who meet the DAWN criteria, mechanical thrombectomy is reasonable within 24 hours of the last known normal [21][22].
Blood Pressure
The guidelines suggest blood pressure management of less than 180/105 mm Hg for the first 24 hours after IV alteplase. A new recommendation is lowering BP initially by 15% in patients with comorbid conditions such as acute heart failure or aortic dissection. There is no benefit of antihypertensive management to prevent death or dependency in patients with BP less than 220/120 mm Hg, who did not receive IV alteplase and have no comorbid conditions requiring blood pressure reduction. This applies to the first 48 to 72 hours after an acute ischemic stroke. For patients with greater than or equal to 220/120 mm Hg who did not receive IV alteplase, the guideline suggests it may be reasonable to reduce BP by 15% in the first 24 hours, although the benefit is uncertain.
Antihypertensive options include:
-
IV labetalol 10 to 20 mg
-
IV nicardipine 5 mg per hour. Increase 2.5 mg per hour every 5 to 15 minutes. The maximum dose is 15 mg per hour
-
Clevidipine 1 to 2 mg per hour IV. Double dose every 15 minutes. Maximum 21 mg per hour
-
Hydralazine, and enalaprilat may be considered
Hypotension and hypovolemia should be avoided because the cerebral perfusion pressure is dependent on the maintenance of elevated MAP as ICP increases due to an ischemic event.
Temperature
Hyperthermia of greater than 38 C should be avoided and treated appropriately. Antipyretics such as acetaminophen may be used. Common sources of infection should be ruled out, such as pneumonia and urinary tract infections. There is insufficient data to support therapeutic hypothermia in acute ischemic strokes currently. A retrospective study recently demonstrated an association between a peak temperature in the first 24 hours of greater than 39 C (100.4 F) and an increased risk of in-hospital mortality.
Glucose
Maintain glucose in the range of 140 to 180 in the first 24 hours. Hypoglycemic patients less than 60 mg/dL should be treated to achieve normoglycemia. The brain is dependent on oxidative pathways that require glucose for metabolism, and the metabolic demand of the brain is high; therefore, episodes of hypoglycemia can decrease the repair of the brain. However, hyperglycemia is hypothesized to decrease reperfusion due to oxidation of nitric oxide-dependent mechanisms and subsequent loss of vascular tone. Moreover, increased acidosis also plays a part, possibly due to injury to lactic acid-sensing channels. Capes et al. showed that hyperglycemia in ischemic stroke patients increases 30-day mortality and is an independent risk factor for hemorrhagic stroke conversion [23].
Nutrition
Early enteric feeding should be encouraged. For patients with dysphagia, use a nasogastric tube to promote enteric feeding. If there is concern that the patient may have swallowing difficulties for a prolonged period (more than 2 to 3 weeks), placing a percutaneous gastrostomy tube is recommended. Early feeding has been demonstrated to have an absolute reduction in the risk of death [24].
DVT Prophylaxis
Intermittent pneumatic compression is recommended for all immobile patients unless there are contraindications. Although prophylactic heparin is often used for immobile patients, the benefit is not clear in stroke patients [25].
Depression Screening
Screening for depression should be considered; however, the optimal timing is unclear.
Cerebellar/Cerebral Edema
Cerebellar edema complicates cerebellar infarctions, and clinicians must be aware that these patients can rapidly decompensate. Cerebellar swelling is thought to be due to cytotoxic and vasogenic edema. The increased intracranial pressure can cause obstructing hydrocephalus on the fourth ventricle, or cause transtentorial herniation of the superior vermis and downward cerebellar tonsillar herniation. Signs include change or worsening mental status, decreased level of consciousness, respiratory abnormalities, change in pupillary size, posturing, and death.
Obtain neurosurgical consult early. A ventriculostomy is indicated in the setting of obstructive hydrocephalus after cerebellar infarct. In cases of cerebral edema with mass effect, a decompressive suboccipital craniectomy is highly recommended. [26][27]
Seizures
If patients experience recurrent seizures, anti-epileptic drugs are recommended. However, the routine prophylactic use of anti-epileptic drugs is not recommended.
Cardiac Evaluation
Cardiac monitoring for atrial fibrillation or other arrhythmias is recommended in the first 24 hours. The benefit of further monitoring is unclear.
An initial troponin is recommended because there is an association between stroke and coronary artery disease.
Antiplatelet Treatment
Aspirin is recommended within 24 to 48 hours of symptom onset. A Cochrane review concluded that aspirin given within 48 hours of symptom onset for ischemic strokes prevented the recurrence of ischemic strokes and improved long-term outcomes. There was no major risk of early intracranial hemorrhage with aspirin [28].
Antithrombotic Treatment
The use of warfarin in secondary stroke prevention is not recommended.
In patients with atrial fibrillation, the guidelines state it is reasonable to initiate oral anticoagulation within 4 to 14 days after neurological symptoms onset.
Statins
High-intensity statins (atorvastatin 80 mg daily or rosuvastatin 20 mg daily) are recommended for patients who are 75 years old or younger and who have clinical atherosclerotic cardiovascular disease. In addition, patients may be continued on statins if they were on them prior to the ischemic stroke.
When to start anticoagulation in patients with atrial fibrillation after acute stroke is always a dilemma. Usually, it depends on various factors like the size of the stroke and other comorbidities. Usually, if the size of the stroke is smaller to moderate, we start anticoagulation in 7-14 days. [29]
Sometimes there are patients with small hemorrhagic transformation after acute stroke, and in this scenario, it is better to wait for anticoagulation for a couple of weeks. This delay is not associated with excessive stroke recurrence. [30]
Differential Diagnosis
Differentials include:
-
Complicated migraines
-
Drug toxicity
-
Intracranial hemorrhage
-
Intracranial tumor
-
Intracranial abscess
-
Hypoglycemia
-
Hyperglycemia
-
Hypertensive encephalopathy
-
Multiple sclerosis
-
Seizure, sepsis
-
Syncope
-
Wernicke encephalopathy
-
Metabolic abnormalities
Prognosis
The prognosis of CVA is highly dependent on the degree, involved structures, the area involved, time of identification and diagnosis, time of initiating treatment, length, and intensity of physical and occupational therapy, and baseline functioning. [31][32]
Complications
In many posterior CVAs, symptomatology can be subtle, and therefore providers should have a low index of suspicion, and they should obtain imaging and neurology consultation earlier.
Postoperative and Rehabilitation Care
Early rehabilitation for stroke patients is beneficial and should be performed, although very early rehabilitation, within 24 hours, should be avoided. The AVERT trial randomized patients to receive very early rehabilitation within 24 hours of stroke compared to usual stroke-unit care, and early mobilization demonstrated less favorable outcomes using the modified Rankin score [33].
Pearls and Other Issues
-
Have a low threshold for evaluation of stroke, especially in at-risk populations.
-
CVA symptoms depend on the ischemic area of the brain and, therefore, can vary.
-
If thrombotic CVA is identified within 4.5 hours of symptom onset, consider alteplase.
-
Be aware of presenting blood pressure. Depending on the type of CVA, blood pressure management recommendations may include aggressive blood pressure management or permissive hypertension.
-
Start an anti-platelet agent within 24 hours of presentation.
-
Consider other risk factor management, including addressing hyperlipidemia, hyperglycemia, and cardiac arrhythmias that may increase the risk of vascular disease or the risk of a thrombotic event.
-
Consider early and aggressive physical and occupational therapy after the onset of CVA.
Enhancing Healthcare Team Outcomes
The decision regarding the diagnosis and management of ischemic stroke is made with an interprofessional team that consists of an emergency department provider, nurse practitioner, neurologist, and radiologist. The current guidelines recommend intravenous alteplase within the first 4.5 hours after the onset of symptoms.
A review of the exclusion criteria for thrombolytics should be performed before administering alteplase. According to the Food and Drug Administration, the contraindications to intravenous thrombolysis include active internal bleeding, recent intracranial surgery or serious head trauma, intracranial conditions that may increase the risk of bleeding, bleeding diathesis, severe uncontrolled hypertension, current intracranial hemorrhage, subarachnoid hemorrhage, and a history of a recent stroke.
The prognosis for patients treated with alteplase is good, but for those who do not receive thrombolytic medication, the outcomes are guarded. [34]
Review Questions
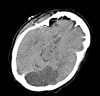
Figure
Figure 5 – An axial section on noncontrast CT head shows left PCA ischemic stroke and no hemorrhage. Contributed by Okkes Kuybu, MD

Figure
Cushing reaction, CNS Ischemic response, cerebrospinal fluid pressure, Compressed brain and arteries, Intracranial tension grows, Cuts off brain blood supply, CNS Ischemic Response starts and arterial pressure rises, Relieved brain ischemia. Contributed (more...)
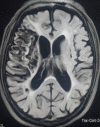
Figure
Encephalomalacia following ischemic stroke. Contributed by Sunil Munakomi, MD
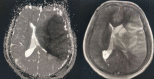
Figure
Left MCA territory infarction. Contributed by Sunil Munakomi, MD
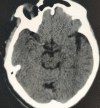
Figure
Right sided MCA 'cord sign' harbingering acute infarction. Contributed by Sunil Munakomi, MD

Figure
Hemorrhagic transformation in a ischemic stroke. Contributed by Sunil Munakomi, MD
References
- 1.
-
Adams HP, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993 Jan;24(1):35-41. [PubMed: 7678184]
- 2.
-
Ntaios G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J Am Coll Cardiol. 2020 Jan 28;75(3):333-340. [PubMed: 31976872]
- 3.
-
Pierik R, Algra A, van Dijk E, Erasmus ME, van Gelder IC, Koudstaal PJ, Luijckx GR, Nederkoorn PJ, van Oostenbrugge RJ, Ruigrok YM, Scheeren TWL, Uyttenboogaart M, Visser MC, Wermer MJH, van den Bergh WM., on behalf of the Parelsnoer Institute-Cerebrovascular Accident Study Group. Distribution of Cardioembolic Stroke: A Cohort Study. Cerebrovasc Dis. 2020;49(1):97-104. [PubMed: 31962331]
- 4.
-
Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB., American Heart Association Statistics Committee. Stroke Statistics Subcommittee. Executive Summary: Heart Disease and Stroke Statistics--2016 Update: A Report From the American Heart Association. Circulation. 2016 Jan 26;133(4):447-54. [PubMed: 26811276]
- 5.
-
Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P., American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation. 2017 Mar 07;135(10):e146-e603. [PMC free article: PMC5408160] [PubMed: 28122885]
- 6.
-
White H, Boden-Albala B, Wang C, Elkind MS, Rundek T, Wright CB, Sacco RL. Ischemic stroke subtype incidence among whites, blacks, and Hispanics: the Northern Manhattan Study. Circulation. 2005 Mar 15;111(10):1327-31. [PubMed: 15769776]
- 7.
-
Kumral E, Bayulkem G, Evyapan D, Yunten N. Spectrum of anterior cerebral artery territory infarction: clinical and MRI findings. Eur J Neurol. 2002 Nov;9(6):615-24. [PubMed: 12453077]
- 8.
-
Brandt T, Steinke W, Thie A, Pessin MS, Caplan LR. Posterior cerebral artery territory infarcts: clinical features, infarct topography, causes and outcome. Multicenter results and a review of the literature. Cerebrovasc Dis. 2000 May-Jun;10(3):170-82. [PubMed: 10773642]
- 9.
-
Cereda C, Carrera E. Posterior cerebral artery territory infarctions. Front Neurol Neurosci. 2012;30:128-31. [PubMed: 22377879]
- 10.
-
Jensen MB, St Louis EK. Management of acute cerebellar stroke. Arch Neurol. 2005 Apr;62(4):537-44. [PubMed: 15824250]
- 11.
-
Aldrich MS, Alessi AG, Beck RW, Gilman S. Cortical blindness: etiology, diagnosis, and prognosis. Ann Neurol. 1987 Feb;21(2):149-58. [PubMed: 3827223]
- 12.
-
Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry. 2005 May;76(5):617-9. [PMC free article: PMC1739623] [PubMed: 15834013]
- 13.
-
Bamford JM, Warlow CP. Evolution and testing of the lacunar hypothesis. Stroke. 1988 Sep;19(9):1074-82. [PubMed: 3046071]
- 14.
-
Lui F, Foris LA, Willner K, Tadi P. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): May 2, 2022. Central Vertigo. [PubMed: 28722891]
- 15.
-
Cassella CR, Jagoda A. Ischemic Stroke: Advances in Diagnosis and Management. Emerg Med Clin North Am. 2017 Nov;35(4):911-930. [PubMed: 28987436]
- 16.
-
Demaerschalk BM, Bobrow BJ, Raman R, Ernstrom K, Hoxworth JM, Patel AC, Kiernan TE, Aguilar MI, Ingall TJ, Dodick DW, Meyer BC., Stroke Team Remote Evaluation Using a Digital Observation Camera (STRokE DOC) in Arizona—The Initial Mayo Clinic Experience (AZ TIME) Investigators. CT interpretation in a telestroke network: agreement among a spoke radiologist, hub vascular neurologist, and hub neuroradiologist. Stroke. 2012 Nov;43(11):3095-7. [PMC free article: PMC3502613] [PubMed: 22984007]
- 17.
-
Johnston KC, Worrall BB., Teleradiology Assessment of Computerized Tomographs Online Reliability Study. Teleradiology Assessment of Computerized Tomographs Online Reliability Study (TRACTORS) for acute stroke evaluation. Telemed J E Health. 2003 Fall;9(3):227-33. [PubMed: 14611689]
- 18.
-
Demaerschalk BM, Kleindorfer DO, Adeoye OM, Demchuk AM, Fugate JE, Grotta JC, Khalessi AA, Levy EI, Palesch YY, Prabhakaran S, Saposnik G, Saver JL, Smith EE., American Heart Association Stroke Council and Council on Epidemiology and Prevention. Scientific Rationale for the Inclusion and Exclusion Criteria for Intravenous Alteplase in Acute Ischemic Stroke: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2016 Feb;47(2):581-641. [PubMed: 26696642]
- 19.
-
Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, Jauch EC, Kidwell CS, Leslie-Mazwi TM, Ovbiagele B, Scott PA, Sheth KN, Southerland AM, Summers DV, Tirschwell DL., American Heart Association Stroke Council. 2018 Guidelines for the Early Management of Patients With Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2018 Mar;49(3):e46-e110. [PubMed: 29367334]
- 20.
-
Tadi P, Lui F. StatPearls [Internet]. StatPearls Publishing; Treasure Island (FL): Apr 12, 2022. Acute Stroke. [PubMed: 30570990]
- 21.
-
Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, Sila CA, Hassan AE, Millan M, Levy EI, Mitchell P, Chen M, English JD, Shah QA, Silver FL, Pereira VM, Mehta BP, Baxter BW, Abraham MG, Cardona P, Veznedaroglu E, Hellinger FR, Feng L, Kirmani JF, Lopes DK, Jankowitz BT, Frankel MR, Costalat V, Vora NA, Yoo AJ, Malik AM, Furlan AJ, Rubiera M, Aghaebrahim A, Olivot JM, Tekle WG, Shields R, Graves T, Lewis RJ, Smith WS, Liebeskind DS, Saver JL, Jovin TG., DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018 Jan 04;378(1):11-21. [PubMed: 29129157]
- 22.
-
Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, Sarraj A, Kasner SE, Ansari SA, Yeatts SD, Hamilton S, Mlynash M, Heit JJ, Zaharchuk G, Kim S, Carrozzella J, Palesch YY, Demchuk AM, Bammer R, Lavori PW, Broderick JP, Lansberg MG., DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018 Feb 22;378(8):708-718. [PMC free article: PMC6590673] [PubMed: 29364767]
- 23.
-
Capes SE, Hunt D, Malmberg K, Pathak P, Gerstein HC. Stress hyperglycemia and prognosis of stroke in nondiabetic and diabetic patients: a systematic overview. Stroke. 2001 Oct;32(10):2426-32. [PubMed: 11588337]
- 24.
-
Dennis M, Lewis S, Cranswick G, Forbes J., FOOD Trial Collaboration. FOOD: a multicentre randomised trial evaluating feeding policies in patients admitted to hospital with a recent stroke. Health Technol Assess. 2006 Jan;10(2):iii-iv, ix-x, 1-120. [PubMed: 16409880]
- 25.
-
AVERT Trial Collaboration group. Efficacy and safety of very early mobilisation within 24 h of stroke onset (AVERT): a randomised controlled trial. Lancet. 2015 Jul 04;386(9988):46-55. [PubMed: 25892679]
- 26.
-
Neugebauer H, Witsch J, Zweckberger K, Jüttler E. Space-occupying cerebellar infarction: complications, treatment, and outcome. Neurosurg Focus. 2013 May;34(5):E8. [PubMed: 23634927]
- 27.
-
Wijdicks EF, Sheth KN, Carter BS, Greer DM, Kasner SE, Kimberly WT, Schwab S, Smith EE, Tamargo RJ, Wintermark M., American Heart Association Stroke Council. Recommendations for the management of cerebral and cerebellar infarction with swelling: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Apr;45(4):1222-38. [PubMed: 24481970]
- 28.
-
Sandercock PA, Counsell C, Tseng MC, Cecconi E. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2014 Mar 26;(3):CD000029. [PMC free article: PMC6669270] [PubMed: 24668137]
- 29.
-
Paciaroni M, Agnelli G, Falocci N, Tsivgoulis G, Vadikolias K, Liantinioti C, Chondrogianni M, Bovi P, Carletti M, Cappellari M, Zedde M, Ntaios G, Karagkiozi E, Athanasakis G, Makaritsis K, Silvestrelli G, Lanari A, Ciccone A, Putaala J, Tomppo L, Tatlisumak T, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D'Amore C, Becattini C, Mosconi MG, Cimini LA, Soloperto R, Masotti L, Vannucchi V, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Sohn SI, Marcheselli S, Mumoli N, De Lodovici ML, Bono G, Furie KL, Tadi P, Yaghi S, Toni D, Letteri F, Tassinari T, Kargiotis O, Lotti EM, Flomin Y, Mancuso M, Maccarrone M, Giannini N, Bandini F, Pezzini A, Poli L, Padovani A, Scoditti U, Denti L, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Gourbali V, Orlandi G, Giuntini M, Chiti A, Giorli E, Gialdini G, Corea F, Ageno W, Bellesini M, Colombo G, Monaco S, Maimone Baronello M, Karapanayiotides T, Caso V. Early Recurrence and Major Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation Treated With Non-Vitamin-K Oral Anticoagulants (RAF-NOACs) Study. J Am Heart Assoc. 2017 Nov 29;6(12) [PMC free article: PMC5779022] [PubMed: 29220330]
- 30.
-
Paciaroni M, Bandini F, Agnelli G, Tsivgoulis G, Yaghi S, Furie KL, Tadi P, Becattini C, Zedde M, Abdul-Rahim AH, Lees KR, Alberti A, Venti M, Acciarresi M, D'Amore C, Mosconi MG, Cimini LA, Altavilla R, Volpi G, Bovi P, Carletti M, Rigatelli A, Cappellari M, Putaala J, Tomppo L, Tatlisumak T, Marcheselli S, Pezzini A, Poli L, Padovani A, Masotti L, Vannucchi V, Sohn SI, Lorenzini G, Tassi R, Guideri F, Acampa M, Martini G, Ntaios G, Athanasakis G, Makaritsis K, Karagkiozi E, Vadikolias K, Liantinioti C, Chondrogianni M, Mumoli N, Consoli D, Galati F, Sacco S, Carolei A, Tiseo C, Corea F, Ageno W, Bellesini M, Colombo G, Silvestrelli G, Ciccone A, Lanari A, Scoditti U, Denti L, Mancuso M, Maccarrone M, Ulivi L, Orlandi G, Giannini N, Gialdini G, Tassinari T, De Lodovici ML, Bono G, Rueckert C, Baldi A, D'Anna S, Toni D, Letteri F, Giuntini M, Lotti EM, Flomin Y, Pieroni A, Kargiotis O, Karapanayiotides T, Monaco S, Maimone Baronello M, Csiba L, Szabó L, Chiti A, Giorli E, Del Sette M, Imberti D, Zabzuni D, Doronin B, Volodina V, Michel P, Vanacker P, Barlinn K, Pallesen LP, Barlinn J, Deleu D, Melikyan G, Ibrahim F, Akhtar N, Gourbali V, Caso V. Hemorrhagic Transformation in Patients With Acute Ischemic Stroke and Atrial Fibrillation: Time to Initiation of Oral Anticoagulant Therapy and Outcomes. J Am Heart Assoc. 2018 Nov 20;7(22):e010133. [PMC free article: PMC6404429] [PubMed: 30571487]
- 31.
-
Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Au R, Kannel WB, Wolf PA. The lifetime risk of stroke: estimates from the Framingham Study. Stroke. 2006 Feb;37(2):345-50. [PubMed: 16397184]
- 32.
-
Carandang R, Seshadri S, Beiser A, Kelly-Hayes M, Kase CS, Kannel WB, Wolf PA. Trends in incidence, lifetime risk, severity, and 30-day mortality of stroke over the past 50 years. JAMA. 2006 Dec 27;296(24):2939-46. [PubMed: 17190894]
- 33.
-
Connell LA, Lincoln NB, Radford KA. Somatosensory impairment after stroke: frequency of different deficits and their recovery. Clin Rehabil. 2008 Aug;22(8):758-67. [PubMed: 18678576]
- 34.
-
Puri I, Bhatia R, Vibha D, Singh MB, Padma MV, Aggarwal P, Prasad K. Stroke-related education to emergency department staff: An acute stroke care quality improvement initiative. Neurol India. 2019 Jan-Feb;67(1):129-133. [PubMed: 30860110]
Source: https://www.ncbi.nlm.nih.gov/books/NBK499997/
0 Response to "Which of the Following Would Lead You to Suspect the Patient s Stroke is Continuing to Get Worse"
Post a Comment